Magnesium alloys are currently lighter metal structural materials. High specific strength, high stiffness, good processing performance, good electromagnetic shielding, good shock absorption, dimensional stability, polishing and castability, good machining, welding, impact resistance, and resistance Aging, rich in raw materials and recyclable, etc. It has broad application prospects in aviation industry, automobile industry, electronic communication and military industry. It is regarded as the ideal electronic product shell material and light vehicle steering system material [1-3]. Because of its light weight and good biocompatibility, it has been considered for implanting biomaterials in the human body [4]. However, its low elastic modulus and poor plasticity, especially poor corrosion resistance, have seriously affected the widespread use of magnesium alloys [5-6]. Magnesium has a relatively negative potential in practical metals, and has a standard electrode potential of -2.73 V. It is easily oxidized and forms a loose porous oxide film spontaneously in air. It is susceptible to corrosion in humid environments, acidic, and neutral media. It is necessary to treat the alloy surface to improve its corrosion resistance. There are many methods for surface treatment of magnesium alloys, such as chemical conversion, chemical oxidation and anodic oxidation, organic coating, surface modification, metal plating, etc., and low-temperature molten salt electroplating aluminum on the surface of magnesium alloy has a good application prospect. Before the electroplating of aluminum, the magnesium alloy must be pre-treated. Usually, a layer of conversion film suitable for electroplating aluminum is obtained by a chemical treatment method. This conversion film temporarily protects the magnesium alloy before electroplating aluminum and prevents magnesium alloys. The surface is exposed to oxidation in the air, and is easily removed or replaced by the plating during the electroplating of aluminum, and the mechanical properties of the conversion film are not excessively required. This experiment mainly studies the pretreatment process of aluminum electroplating on magnesium alloys in order to find a simple and effective pretreatment method for electroplating aluminum on the surface of magnesium alloys.
1 Test section
1.1 Materials used in the test
The test material used was AZ261 magnesium alloy. The mass fraction of each alloying element was: w(Al)=5.0%~7.0%, w(Zn)=0.8%~1.0%, w(Fe)<0.01%, w (Si)<0.01%, w(Cu)<0.03%, w(Ni)<0.005%, remainder Mg.
1.2 Process flow
Samples → Polishing → Degreasing → Washing → Alkali washing → Washing → Passivation → Washing → Drying → Drying → Detection.
1.3 Pretreatment Process Parameters and Formulas
(1) Sample preparation. Manufacture each 20mm × 10mm × 5mm AZ61 magnesium alloy specimen, sandblast the surface with 800#, 1200#, and 1500# water sandpaper in succession, then polish the surface to ensure that all specimens have the same Surface roughness.
(2) Degreasing. The polished sample is placed in an acetone solution and cleaned ultrasonically for 5 minutes. The purpose of the degreasing is to remove oil, dirt, polishing paste, etc. from the surface of the workpiece and obtain a clean, grease-free surface.
(3) Alkaline wash. It was kept in a 100 g/L aqueous solution of sodium hydroxide at 70-80° C. for 10 min to further remove the oily surface of the sample. In an alkaline solution, the surface oxide film of magnesium is converted and MgO becomes Mg(OH)2.
(4) passivation. Prepare different concentrations of HF (volume fraction of 40%, analytically formulated) at room temperature for two-factor three-level full factorial test, consider the impact of HF on the passive film from both concentration and time. The process parameters are shown in Table 1.
Table 1 Test Factor Level Value
Time t/min HF concentration/mL·(100mL)-1 27.5 15 2.5 1 1# 4# 7# 5 2# 5# 8# 10 3# 6# 9#
1.4 Detection of Conversion Film Performance
Detection of the pre-treated conversion film includes:
(1) Phase XRD test. The structure and the phase of the pre-treated conversion film layer were analyzed by X-ray diffraction.
(2) Test of film thickness. The thickness of the pre-treated conversion film was measured using a eddy current thickness meter model detekrophysik-minitest600BN2 produced in Germany.
(3) Surface morphology observation and analysis. The metallographic microscope and JSM-6700F/INCA-ENERGY model field emission scanning electron microscope/energy dispersive spectrometer were used to analyze the surface morphology and energy spectrum of the treated conversion film.
(4) Test of corrosion resistance. The effect of MgF2 conversion coating on the corrosion resistance of magnesium alloy surface was studied by potentiodynamic polarization method. The test cell of the potentiodynamic polarization test curve is the M273A system. The electrochemical measurement system adopts a three-electrode system, with a saturated calomel electrode (SCE) as a reference electrode and a Pt electrode as an auxiliary electrode. The study sample is a working electrode. The test was performed at room temperature (about 25°C) and the sample was stabilized in an aqueous solution of w(NaCl)=3.5% for about 30 minutes. Polarization tests were started and the test was performed at a scan rate of 10 mV/s.
2 test results and analysis
2.1 XRD test results
The result of the XRD test of the AZ61 magnesium alloy after the fluorination treatment is shown in FIG. 1 , and the main components are Mg and MgF2. It shows that after the pretreatment, the main component of the magnesium alloy surface is magnesium fluoride, and a magnesium fluoride conversion film is formed on the surface of the magnesium alloy.
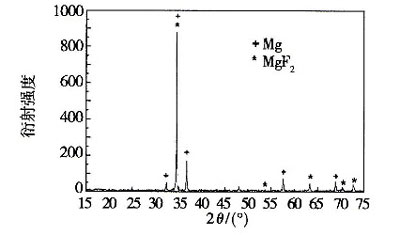
Figure 1 XRD test results
2.2 Film Thickness Test Results
It can be seen from Table 2 that the magnesium fluoride conversion film obtained from the pretreatment of the 2#, 9#, 3# and 7# samples is relatively thick, while the conversion films obtained from the 4#, 6# and 1# are relatively thin.
2.3 Observation and Analysis of Specimen After Treatment
Using JSM-6700F/INCA-ENERGY model field emission scanning electron microscopy and metallographic microscope, the surface of the pre-treated conversion film was observed by electron microscopy and fracture surface morphology. Figure 2 shows the surface scan morphology of the sample after hydrofluoric acid treatment. Figure 3 shows the metallographic profile.
It can be seen from the surface scanning topography image 2 that a magnesium fluoride film is formed on the surface of the treated sample, but the surface morphology of the film formed by different processing parameters is different. After the treated sample was left for a period of time, the better surface of the sample was clean and the "cluster" oxides covered less; while the surface of the poor samples had more "cluster" oxides. This shows that the better magnesium fluoride conversion film obtained after hydrofluoric acid treatment has better oxidation resistance, has a better protective effect on the surface of the magnesium alloy before electroplating, and meets the requirements of pre-plating treatment. The illustrated topography is a typical “dry river bed topographyâ€. Cracks are formed due to natural shrinkage of the generated fluoride after it has been placed for a period of time. The existence of cracks will have a certain effect on the protection effect for a long time, but since the workpieces will be electroplated immediately after treatment, they will not be placed for a long time, so the impact is not significant. At the same time, the presence of tiny gaps facilitates the intrusion of molten salt during electroplating, which facilitates the exfoliation of the passivation film and is replaced, and will provide convenient conditions for the electrodeposition of aluminum.
Table 2 Sample film thickness test results
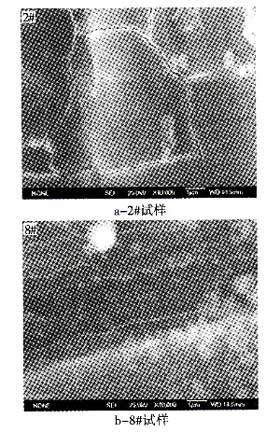
Fig. 2 Surface morphology of AZ261Mg alloy sample after treatment
FIG. 3 is an optical metallographic photograph of a magnesium alloy magnesium fluoride film cross-section. In the figure, I is a mosaic material, II is a magnesium fluoride film, and III is a magnesium alloy matrix. It can be clearly seen that, when treated with a low concentration of hydrofluoric acid, the magnesium alloy matrix is ​​heavily corroded and the surface is serrated, showing corrosion characteristics. The high concentration of hydrofluoric acid treatment, the corrosion is lighter, the film is thicker and the surface is smoother, showing passivation characteristics, in line with the requirements of the pre-treatment.
2.4 Test of corrosion resistance
As can be seen from Table 3, the corrosion potential of the samples after treatment is significantly higher than the corrosion potential of the untreated AZ61 magnesium alloy. This shows that the MgF2 conversion film obtained by the hydrofluoric acid treatment improves the magnesium alloy in the NaCl solution. In the stability, its corrosion resistance is better than the untreated AZ61 magnesium alloy, in which the corrosion potentials of the 2#, 9#, and 7# samples are higher, indicating that the corrosion resistance is better than that of other samples. The corrosion mechanism of AZ61 magnesium alloy in NaCl solution is: the active Clˉ adsorbs on the surface film of the sample and replaces the oxygen in the surface film to form soluble magnesium chloride. The MgF2 conversion film obtained by the treatment replaces the oxide film on the surface of the substrate. Since fluorine has strong electronegativity, it is harder to be replaced by chlorine than oxygen, and effectively improves the corrosion resistance of the substrate.
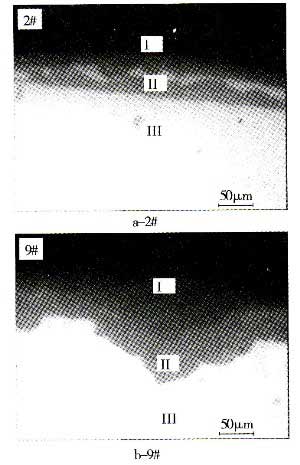
Fig. 3 Cross-sectional morphology of the treated sample
Table 3 Self-corrosion potentials of AZ61 magnesium alloy specimens
Centrifuge tubes are used in laboratory centrifuges, machines that spin samples in order to separate solids out of liquid chemical solutions. The centrifuge tubes can be made of glass or plastic, and resemble miniature test tubes with tapered tips. They are mainly used in series test like the centrifuge of nucleic acid

50 ml Centrifuge Tubes,15 ml Centrifuge Tubes,5ml Centrifuge Tubes,Centrifuge Tube Holder,Micro centrifuge Tubes
Yong Yue Medical Technology(Kunshan) Co.,Ltd , https://www.yongyuemedicals.com