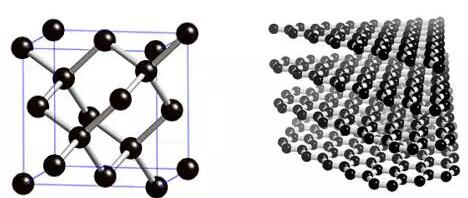
Both diamond and charcoal are natural minerals. Diamonds are found in cooled craters and are generally crystalline (pig, pink or yellowish green) blocks that are angular and hard (Figure 1). Most importantly, diamonds are very rare and their mass units are carats (1 carat = 0.2 g). Because of its crystal clear, non-destructive and rare characteristics, diamond is worthy of the city. It is a symbol of wealth, and sometimes even equals love. Today we know that almost all properties of diamond are extreme values, such as its thermal conductivity (which can be used as a heat sink component for high-power devices, the study of diamond specific heat is the key to developing solid quantum theory), and the maximum modulus of elasticity. And the temper is also the biggest (replacement of one carbon atom in diamond with a nitrogen atom may smother another carbon atom, the electronic state of this impurity-hole pair is said to be entangled, can be used to make quantum computers), and so on. In contrast, charcoal will form large mines with reserves of hundreds of millions of tons. Charcoal is not uplifting, there is no formality, it is dark, and it is black. The charcoal looks good in graphite, the bright metallic color (Figure 1). According to solid state physics, graphite is a semi-metal or indirect bandgap semiconductor with an energy gap of -0.04 eV, so it is electrically conductive. Graphite has a layered structure that is easy to cleave and leave debris. It is conceivable that although carbon (graphite) has important applications, such as burning fire, pencil core and battery electrode, its value has never been higher. Before Antoine Lavoisier burned diamonds in 1772 and noticed that diamonds were charcoal, charcoal never thought of having a rich relative of diamonds. It is today, if you dare to tell a lady that the diamond on her diamond ring is the briquettes of your home, she will also directly drop the diamond ring on your face.

Shortly after the combustion reaction proved that the diamond was charcoal, the French chemist Guyton de Mouveau said that the diamond was directly converted into graphite in 1799, and the diamond and charcoal were definitely certain. Burning diamonds into smoke and directly turning into graphite are all actions of losing the family, and people can't help but shake their heads. But if it turns out to turn graphite into diamonds, how exciting it is, the ladies on the street can wear a diamond ring with two pounds of diamonds. What the scientific historians may not pay much attention to is that the point of graphite into diamond is a more tempting career than the stone. The former's lure to scientists and the promotion of science are not inferior to the latter.
In order to advance science and gain wealth, many scientists have devoted themselves to the business of graphite into diamonds. In 1880, the British JB Hannay declared that paraffin, bone oil and lithium metal were calcined in steel pipes to obtain diamond particles. This work has been highly praised and sought after by other scientists, but it has been found that the results in Han can not be repeated, and diamonds are not obtained by this method. However, the enthusiasm of scientists will not have the slightest impact, more people are committed to this great cause, and more thinking and methods are being tested one by one in different laboratories.
3 Mowassan successfully synthesized diamond
The French chemist Henri Moissan (1852-1907) also joined the team of dream diamonds into diamonds. Mowasan is a great chemist who won the 1906 Nobel Prize in Chemistry for the separation of elemental fluorine from compounds. Mowasang has a great interest in diamonds. In 1893, natural silicon carbide (SiC) in meteorites was mistaken for diamonds, so that natural silicon carbide is now called moissanite (today, there are still people or unintentional or Deliberately consider SiC as diamond or other new type of silicon carbide). Movasan thought that to convert graphite into diamond, high pressure is a necessary condition. He noticed that there may be carbon particles in the molten iron. If the high-temperature molten iron is rapidly cooled, the stress formed by the local strain may achieve the pressure condition of transforming graphite, thereby obtaining diamond. It should be said that this idea is very correct. With the jargon of the fund today, the technical route is clear and reasonable.
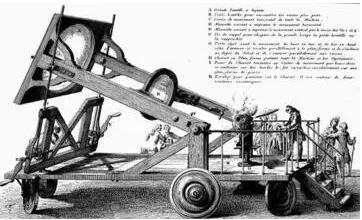
Mowassan happened to be an expert in electric arc furnaces. In order to obtain high temperature (~3500 °C), Moissan improved the electric arc furnace and smelted molten iron in person using an electric arc furnace (Fig. 4). In 1893, Movasan was acid-washed in a molten iron system after melting to obtain diamond particles.

After the realization of synthetic diamond, Movasan did not transfer his synthetic technology to the production stage, which is very strange from the vulgar point of profit. However, at the beginning, everyone did not think too much, because Mowasan is indeed a scientist who has a wide range of interests and can ride freely in a wide range of scientific fields. He has too many chemistry research directions to display his talents. In 1906, Mowassan won the Nobel Prize in Chemistry, and his opponent was the famous Mendeleev.
However, those who follow the technical route of Mowasang to synthesize diamonds but have nothing to gain have become increasingly impatient. After the death of Mowasan in 1907, the impatience of these people became a bit unscrupulous. The test confirming and repeating Mowasan turned to "falsification", and some people may directly cast doubts on Mowasan’s will. Later, Mowasang’s widow was really troubled. He finally admitted that Mowasang’s assistant was really bored with endless repeated experiments, and he had to deal with Mowasang and secretly put it at home. The diamond particles in the room were mixed (I guess) the broken charcoal iron scraps to fool Mowasang. In other words, Mowasang did not know that he was cheated by his assistant.
To do a simple arithmetic, from the declaration of diamonds in 1893 to the death of Mowasan in 1907, this is about 14 years. For 14 years, the scientific significance of synthetic diamonds and the fame and fortune brought about it, can Mowasang really ignore this experiment? Even if he doesn't care about fame and fortune, those scientists who care about fame and fortune and fail to get diamonds will not be able to meet with him and will ask face to face. Even if the assistant has been doing experiments, how can the assistants get so many diamonds? It seems hard to be convinced to blame this big scam on a nameless assistant.
The lesson that this story teaches us is that blaming academic misconduct on students or low-level researchers is a routine in the academic world. As a student or a low-level researcher, even if you have a lot of hardships in your career, you can't fake others. Take the chestnuts for others, the chestnuts are not yours, but the hands of the fire are yours.
Author Cao Zexian (Researcher, Institute of Physics, Chinese Academy of Sciences)
Comment
[1] The introduction of the word "carbon" is purely for the Chinese to ask for trouble. China's coal chemistry research institute can only study carbon chemistry, which is too good. This word should be permanently removed from the Chinese dictionary!
Nonwoven geotextiles are made of polyester or polypropylene in random directions and punched together by needles. Geotextiles has good impermeability and resistance to deformation, which allows geotextiles be widely applied in civil projects for separation, filtration, reinforcement, protection and drainage.

Specification:
(1). 80g/m2~1000g/m2
(2). 1m~8m in roll width, the length as client's request.
(3). Color: white , black , green or at your request
Property:
(1). Good flexibility, resistant to corrosion, resistant to acids and alkali, anti-oxidation
(2). Separation, filtration, drainage, reinforcement , protection and maintentance function.

| Gram weight | Thickness(mm) | Breaking strength(kn/m) | CBR burst strength(KN) | Elongation at break(% | Tear Strength(KN) | Sieve size O99(O35)mm |
| 100 | 0.9 | 2.5 | 0.3 | 25-100 | 0.08 | 0.07-2.0 |
| 150 | 1.3 | 4.5 | 0.6 | 25-100 | 0.12 | 0.07-2.0 |
| 200 | 1.7 | 6.5 | 0.9 | 25-100 | 0.16 | 0.07-2.0 |
| 250 | 2.1 | 8 | 1.2 | 25-100 | 0.2 | 0.07-2.0 |
| 300 | 2.4 | 9.5 | 1.5 | 25-100 | 0.24 | 0.07-2.0 |
| 350 | 2.7 | 11 | 1.8 | 25-100 | 0.28 | 0.07-2.0 |
| 400 | 3 | 12.5 | 2.1 | 25-100 | 0.33 | 0.07-2.0 |
| 450 | 3.3 | 14 | 2.4 | 25-100 | 0.38 | 0.07-2.0 |
| 500 | 3.6 | 16 | 2.7 | 25-100 | 0.42 | 0.07-2.0 |
| 600 | 4.1 | 19 | 3.2 | 25-100 | 0.46 | 0.07-2.0 |
| 800 | 5 | 25 | 4 | 25-100 | 0.6 | 0.07-2.0 |
Shandong Jinruixiang geotextile material Co.,Ltd
Shandong Famous Brand
Certificate of Inspection of Quality:CRCC Certification
Enterprise of Abiding by Strictly Contract and Being Trustworthy
ISO9001:2008 Quality Management System Certification; National Industrial Production License.




1.Water conservancy project and hydropower projects
2.Road paving, railway
3.Airport and port
4.River band protection and tunnel
5. Environmental protectiom, etc


Packaging Details:
Nonwoven Geotextile is packed in the woven bags or follow your demand.
Keep it away from moisture and fire!!!
Delivery Detail:
Generally within 5-15 days after receipt 30%T/T deposit !


Pp Geotextile,Geotextile Non Woven,Hybrid Nonwoven Geotextiles,Geotextile Non Woven Fabric
Shandong Jinruixiang Geotextile Material Co., Ltd , https://www.jrxgeosynthetics.com